Abstract
Background:
Prothrombin time (PT) and activated partial thromboplastin time (aPTT) are screening tests of hemostasis commonly ordered for anticoagulant therapy monitoring, evaluation of bleeding, screening for acquired or inherited deficiencies of coagulation factors, and preoperative screening. Evaluation of a prolonged PT/aPTT is best accomplished in an algorithmic fashion consisting of mixing studies and factors assays or inhibitor testing as indicated. However, there is little data available on frequency of conditions that may result in abnormalities of PT/aPTT. The Special Coagulation Laboratory (SCL) at our institution offers an algorithmic approach termed Prolonged PT/aPTT Profile (PT/aPTT profile).
Aim:
To review our institutional experience with PT/aPTT profile testing (indications and outcomes), and to provide clinical correlation of abnormal PT/aPTT tests on patients seen at our institution in an effort to explain common causes of altered hemostatic testing in outpatient clinical practice.
Methods:
In this retrospective study, following IRB approval, we reviewed the medical records of patients who underwent outpatient PT/aPTT profile testing in the SCL at Mayo Clinic in Rochester, Minnesota between 2010 and 2017. Data abstracted included patient demographics and clinical presentation, indications for testing, use of anticoagulants, medical diagnoses which could potentially influence coagulation, and results of hemostatic testing (Table 1). Statistical analyses were completed using SAS 9.4 (SAS Institute Inc., Cary, North Carolina). Continuous variables are presented as median (range) while discrete variables are summarized as frequency (percentage). Comparisons of clinical characteristics between groups are made using the rank-sum test for continuous variables. Statistical significance is defined as a 2-tailed P value of less than 0.05.
Results:
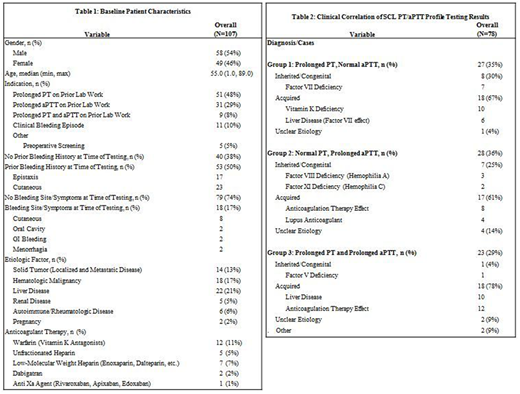
A total of 107 patients met our study criteria, 58 (54 %) male, median age 55 years (range 1-89). Indications for testing included prior prolongation of PT (48%), PTT (29%), PT and aPTT (8%) on outside coagulation testing performed in a non-SCL setting, clinical bleeding episodes (10%) and preoperative screening (5%). Patients with established medical diagnoses known to influence coagulation testing including solid tumors, hematologic malignancies, liver disease, renal disease, autoimmune and rheumatologic conditions and pregnancy were documented along with other baseline patient characteristics (Table 1).
SCL PT/aPTT profile testing revealed normal results in 29 of 107 (27%) patients on repeat testing while 78 patients warranted further evaluation of which 27 (35%) had an isolated prolonged PT, 28 (36%) had a prolonged APTT and 23 (29%) had prolongations of both PT and aPTT.
After further workup and clinical correlation, causes of abnormal outpatient PT/aPTT profile testing were found to be related to an underlying congenital condition in 16 (21%) patients - most commonly factor VII deficiency (9%) and factor VIII deficiency (4%) - acquired conditions in 53 (68%) patients - most commonly anticoagulant therapy effects (31%) and liver disease (21%) - and of unclear etiology in 7 (9%) cases (Table 2).
Specifically, the most common cause of an isolated prolonged PT in our study was found to be vitamin K deficiency (37%) whereas elevations in aPTT and both PT/aPTT were most frequently attributed to the effects of anticoagulant therapy (29% and 52% respectively). Other causes of prolongation on PT/aPTT profile testing following clinical correlation were also identified (Table 2).
Discussion:
While PT and aPTT are commonly ordered tests, there is little evidence regarding epidemiology of testing indications, distribution of test results, and the clinical correlation of test results. Our study demonstrates that abnormalities in hemostatic testing can be attributed to a wide range of causes including artefactual prolongation influenced by pretest variables or marked abnormalities in secondary hemostasis due to inherited and acquired conditions. Strengths of this study include the SCL algorithmic approach to testing to further elucidate coagulation cascade abnormalities to properly characterize conditions that pose severe hemostatic risk while limitations of this study reflect the experience of a single institution and possible referral bias.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.